ReachMD
Be part of the knowledge.™Biosimilar News: Samsung Bioepis, Sandoz to Develop SB17, a Proposed Stelara Biosimilar to Stelara - Practical Dermatology
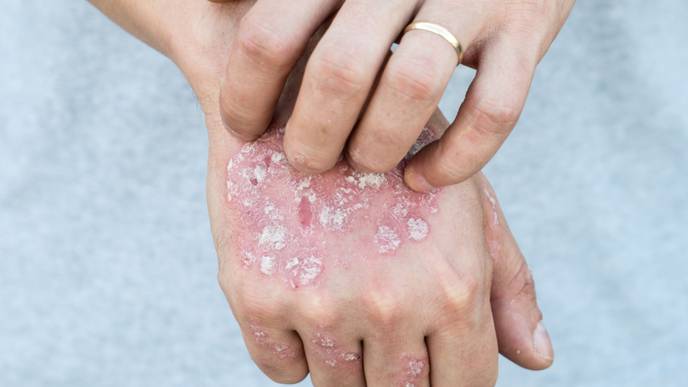
Samsung Bioepis Co., Ltd. has entered into a commercialization agreement with Sandoz for SB17, a proposed biosimilar to Stelara (ustekinumab).
Samsung Bioepis will be responsible for development, regulatory registration and manufacture and supply of the product in the United States (US), Canada, European Economic Area (EEA), Switzerland and United Kingdom (UK), and Sandoz will be responsible for commercialization in these regions.
SB17, a proposed biosimilar to Stelara (ustekinumab), is Samsung Bioepis’ fourth candidate in immunology pipeline. SB17 Phase 3 clinical study results are set to be presented at a medical congress later this year.
In March, the company presented Phase 1 clinical study results of SB17 at the 2023 American Academy of Dermatology Annual Meeting. The study demonstrated pharmacokinetics (PK) equivalence and comparable safety, tolerability, immunogenicity profiles between SB17 and reference ustekinumab.
Facebook Comments